Full window version (looks a little nicer). Click <Back>
button to get back to small framed version with content indexes.
This material (and images) is copyrighted!
See my copyright
notice for fair use practices.
This chapter covers all of the solar system that is not the planets or the
Sun: meteorites, asteroids, and comets. I group them all together as ``solar
system fluff'' because the objects are much smaller than planets and most moons.
The ``fluff'' may be small in size but certainly not in importance. We get clues
of the origin of the solar system from these small objects.
There are thousands, even millions, of small
rocks that orbit the Sun, most of them between the orbits of Mars and Jupiter.
A plot of the
known asteroids is available at the Minor Planet Center. About
one million of them are larger than 1 kilometer across. Those smaller than about
300 kilometers across have irregular shapes because their internal gravity is
not strong enough to compress the rock into a spherical shape. The largest
asteroid is Ceres with a diameter of 1000 kilometers. Pallas and Vesta have
diameters of about 500 kilometers and about 15 others have diameters larger than
250 kilometers. The number of asteroids shoots up with decreasing size. The
combined mass of all of the asteroids is less than the Moon's mass. Very likely
the asteroids are pieces that would have formed a planet if Jupiter's strong
gravity had not stirred up the material between Mars and Jupiter. The rocky
chunks collided at speeds too high to stick together and grow into a planet.
Though there are over a million asteroids, the volume of space they inhabit
is very large, so they are far apart from one another. Unlike the movie The
Empire Strikes Back, where the spacecrafts flying through an asteroid belt
could not avoid crashing into them, real asteroids are at least tens of
thousands of kilometers apart from each other. Several spacecraft sent to the
outer planets have travelled through the asteroid belt with no problems.
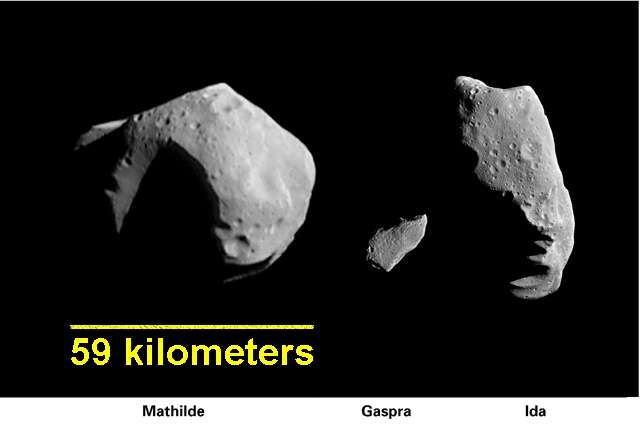
Two of the three types of asteroids are represented by the asteroids that
have been explored up close with spacecraft. Mathilde (left) is a dark C-type
(brightness enhanced several times to match the other two). Gaspra and Ida are
S-type asteroids.
There are three basic types of asteroids:
- C: they are carbonaceous---made of silicate materials with a lot
of carbon compounds so they appear very dark. They reflect only 3 to 4% of the
sunlight hitting them. You can tell what they are made of by analyzing the
spectra of sunlight reflecting off of them. This reflectance spectra shows
that they are primitive, unchanged since they first solidified about
4.6 billion years ago. A sizable fraction of the asteroids are of this type.
The asteroid called Mathilde, recently explored by the NEAR spacecraft is an
example of this type (see picture above).
- S: they are made of silicate materials without the dark carbon
compounds so they appear brighter than the C types. They reflect about 15 to
20% of the sunlight hitting them. Most of them appear to be primitive and they
make up a smaller fraction of the asteroids than the C types. Gaspra and Ida,
explored by the Galileo spacecraft on its way to Jupiter, are examples of this
type (see picture above).
- M: they are made of metals like iron and nickel. These rare type
of asteroids are brighter than the S and C types. We think they are the
remains of the cores of differentiated objects. Large objects were hot
enough in the early solar system so that they were liquid. This allowed the
dense materials like iron and nickel to sink to the center while the lighter
material like ordinary silicate rock floated up to the top. Smaller objects
cooled off quicker than larger objects, so they underwent less
differentiation. In the early solar system, collisions were much more common
and some of the smaller differentiated large asteroids collided with one
another, breaking them apart and exposing their metallic cores.
A few
other asteroids have surfaces made of basalt from volcanic lava flows. When
asteroids collide with one another, they can chip off pieces from each other.
Some of those pieces, called meteoroids, can get close to the Earth and
be pulled toward the Earth by its gravity.
The quick flashes of light in the sky most
people call ``shooting stars'' are meteor---pieces of the rock glowing
from friction with the atmosphere as they plunge toward the surface. Most of the
meteors you see are about the size of a grain of sand. More meteors are seen
after midnight because your local part of the Earth is facing the direction of
its orbital motion around the Sun. Meteoroids moving at any speed can hit the
atmosphere. Before midnight your local part of the Earth is facing away from the
direction of orbital motion, so only the fastest moving meteoroids can catch up
to the Earth and hit the atmosphere. The same sort of effect explains why an
automobile's front windshield will get plastered with insects while the rear
windshield stays clean.
If the little piece of rock makes it to the surface without burning up, it is
called a meteorite. There are three basic types of meteorites.
- Stones: they are made of silicate material
with a density around 3 (relative to the density of water) and look like
ordinary Earth rocks. This makes them hard to distinguish from Earth rocks so
they don't stand out. About 95 to 97% of the meteorites are of these type.
About 85% of the stones are primitive, unchanged since they first
solidified about 4.6 billion years ago. Most of the primitive stones have
chondrules---round glassy structures 0.5 to 5 millimeters across
embedded in the meteorites. They are solified droplets of matter from the
early solar nebula and are the oldest part of a primitive meteorite.

A carbonaceous meteorite
containing chondrules (courtesy of the Planetary Data Center).
The oldest of the stone meteorites are the carbonaceous meteorites.
They contain silicates, carbon compounds (giving them their dark color), and a
surprisingly large amount of water (about 22%). They are probably chips of
C-type asteroids. Some of the carbonaceous meteorites have organic molecules
called amino acids. Amino acids can be connected together to form proteins
that are used in the biological processes of life. There is the possibility
that meteorites like these may have been the seeds of life on the Earth. In
addition, these type of meteorites may have provided the inner planets with a
lot of water. The terrestrial planets may have been so hot when they formed
that most of the water in them at formation evaporated away to space. The
impact of millions to billions of carbonaceous meteorites in the early solar
system may have replenished the water supply on the terrestrial planets.
About 10 to 12% of the stones are from the crust of differentiated parent
objects. Therefore, they are younger (only 4.4 billion years old). The
lighter-colored stones are chips from the S-type of asteroids.
- Stoney-Irons: only 1% of the meteorites are of
this type. They have a variable mixture of metal (iron and nickel) and rock
(silicates) and have densities ranging from 4 to 6 (relative to the density of
water). They come from a differentiated object at the boundary between the
metal core and the rock crust. They are 4.4 billion years old.
- Irons: although they make up about 40% of the
meteorites found worldwide, only 2 to 3% of the meteorites are these type.
They make up so many of the ones found because they are easily distinguished
from Earth rocks. They are noticeably denser than Earth rocks, they have a
density around 7. They come from the core of a differentiated body and are
made of iron and nickel. They are 4.4 billion years old. Irons sometimes have
large, coarse-grained crystalline patterns (``Widmanstatten patterns'') that
is evidence that they cooled slowly.

An iron meteorite
(courtesy of the Planetary Data
Center).
The primitive meteorites are probably the most
important ones because they hold clues to the composition and temperature in
various parts of the early solar nebula.
Most stoneys look like Earth rocks
and so they are hard to spot. The rare irons are easy to distinguish from Earth
rocks and make up most of the ones found worldwide. Usually the meteorites
science museums show off are iron meteorites. Not only does their high density
and metal composition set them apart from ordinary rocks, the iron meteorites
are stronger. This means they will more likely survive the passage through the
atmosphere in one piece to make impressive museum displays. The stone meteorites
are more fragile and will break up into several pieces (less impressive for
museum displays).
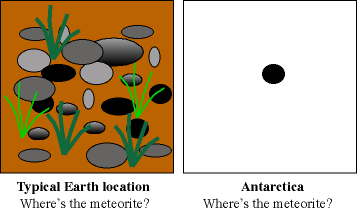
To get an accurate number for the proportion of meteorites that fall to the
Earth (an unbiased sample), meteorite searchers go to a place where all
types of rocks will stand out. The best place to go is Antarctica where the
stable, white ice pack makes darker meteorites easy to find. Meteorites that
fell thousands of years ago can still be found in Antarctica without significant
weathering. Since the 1980's thousands of meteorites have come from here. For
further exploration, check out the Antarctica
Meteorite web site at the Planetary Materials
Curation office of NASA and the ANSMET site at Case
Western Reserve University.

Meteorites stand out against
the snow and ice background of Antarctica.
Most meteorites are pieces of asteroids, but a few are from the Moon. A
select few, the Shergotty-Nakhla-Chassigny (SNC) meteorites, may be
from Mars. The relative abundances of magnesium and heavy nitrogen (N-15) gases
trapped inside the SNC meteorites is similar to the martian atmosphere as
measured by the Viking landers and unlike any meteorites from the asteroids or
Moon. Also, the isotope ratios of argon and xenon gas trapped in the meteorites
most closely resemble the martian atmosphere and are different than the typical
meteorite. The analysis of the soil and rocks by the Mars Pathfinder confirm this.

A meteorite blasted from
Mars.
Most SNC meteorites are about 1.4 Gyr old, but the one with suggestions of
extinct Martian life is about 4.5 Gyr old. The discovery was published in
the August 16, 1996 issue of the journal Science.
Non-subscribers can find a copy of the article here.
Recent studies of the meteorite have cast considerable doubt on the initial
claims for fossil microbes in the rock. There is strong evidence of
contamination by organic molecules from Earth, so this meteorite does not
provide the conclusive proof hoped for. A detailed description of SNC meteorites
is given at http://www.jpl.nasa.gov/snc.
There are several ways to figure out
relative ages, that is, if one thing is older than another. For example, looking
at a series of layers in the side of a cliff, the younger layers will be on top
of the older layers. Or you can tell that certain parts of the Moon's surface
are older than other parts by counting the number of craters per unit area. The
old surface will have many craters per area because it has been exposed to space
for a long time. But how old is ``old''? If you assume that the impact rate has
been constant for the past several billion years, then the number of craters
will be proportional to how long the surface is exposed. However, the crater
number relation must be calibrated against something with a known age.
To measure the passage of long periods of time, scientists take advantage of
a regularity in certain unstable atoms. In radioactive atoms the nucleus will
spontaneously change into another type of nucleus. When looking at a large
number of atoms, you see that a certain fraction of them will change or
decay in a certain amount of time that depends on the type of
atom---more specifically, the type of nucleus. Radioactive dating is an
absolute dating system because you can determine accurate ages from the
number of remaining radioactive atoms in a rock sample. Most of the radioactive
isotopes used for radioactive dating of rock samples have too many
neutrons in the nucleus to be stable.
An isotope is a particular form of an element. All atoms of an element
have the same number of protons in their nucleus and behave the same way in
chemical reactions. The atoms of an isotope of a given element
have same number of protons AND neutrons in their nucleus. Different isotopes of
a given element will have the same chemistry but behave differently in
nuclear reactions. In a radioactive decay, the original radioactive
isotope is called a parent isotope and the resulting isotope after the
decay is called a daughter isotope. For example, Uranium-238 is the
parent isotope that breaks apart to form the daughter isotope Lead-204.
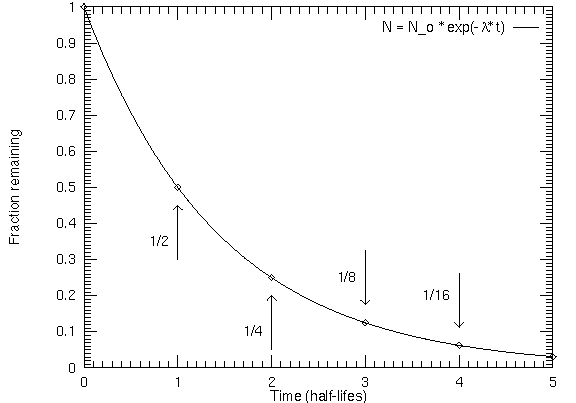
Radioactive isotopes will decay in a regular exponential way such that
one-half of a given amount of parent material will decay to form daughter
material in a time period called a half-life. A half-life is NOT
one-half the age of the rock! When the material is liquid or gaseous, the parent
and daughter isotopes can escape, but when the material solifies, they cannot so
the ratio of parent to daughter isotopes is frozen in. The parent isotope can
only decay, increasing the amount of daughter isotopes. Radioactive dating gives
the solidification age.
There are two simple steps for radioactive dating:
- Find out how many times you need to multiply (1/2) by itself to get the
observed fraction of remaining parent material. Let the number of the times be
n. For example 1/8 = (1/2) × (1/2) × (1/2), so n = 3. The number
n is the number of half-lives the sample has been decaying. If
some material has been decaying long enough so that only 1/4 of the
radioactive material is left, the sample is 2 half-lives old: 1/4 = (1/2) ×
(1/2), n =2.
- The age of the sample in years = n × (one half-life in years).
How do you do that?If 1/8 of the original amount of parent
isotope is left in a radioactive sample, how old is the sample?
Answer: After 1 half-life, there is 1/2 of the original amount of
the parent left. After another half-life, there is 1/2 of that 1/2 left =
1/2 × 1/2 = 1/4 of original amount of the parent left. After yet another
half-life, there is 1/2 of that 1/4 left = 1/2 × 1/2 × 1/2 = 1/8 of the
original amount of the parent left (which is the fraction asked for). So
the rock is 1 half-life + 1 half-life + 1 half-life = 3 half-lives old (to
get the age in years, simply multiply 3 by the half-life in years).
|
If you have a fraction that is not a multiple of 1/2, then it is more
complicated. The age = [ln(original amount of parent material / current
amount of parent material) / ln(2)] × (half-life in years), where
ln() is the ``natural logarithm'' (it is the ``ln'' key on a scientific
calculator).
There are always a
few astronomy students who ask me the good question (and many others who are too
shy to ask), ``what if you don't know the original amount of parent material?''
or ``what if the rock had some daughter material at the very beginning?'' The
age can still be determined but you have to be more clever in determining it.
One common sense rule to remember is that the number of parent isotope atoms
+ the number of daughter isotope atoms = an unchanging number throughout time.
The number of parent isotopes decreases while the number of daughter isotopes
increases but the total of the two added together is a constant. You need to
find how much of the daughter isotopes in the rock (call that isotope ``A'' for
below) are not the result of a radioactive decay of parent atoms. You
then subtract this amount from the total amount of daughter atoms in the rock to
get the number of decays that have occurred since the rock solified. Here are
the steps:
- Find another isotope of the same element as the daughter that is
never a result of radioactive decay (call that isotope ``B'' for
below). Isotopes of a given element have the same chemical properties, so a
radioactive rock will incorporate the NONradioactively derived proportions of
the two isotopes in the same proportion as any nonradioactive rock.
- Measure the ratio of isotopes A and B in a nonradioactive rock. This
ratio, R, will be the primitive (initial) proportion of the two
isotopes.
- Multiply the amount of the non-daughter isotope (isotope B) in the
radioactive rock by the ratio of the previous step: (isotope B) × R =
initial amount of daughter isotope A that was not the result of decay.
- Subtract the initial amount of daughter isotope A from the rock sample to
get the amount of daughter isotope A that IS due to radioactive decay. That
number is also the amount of parent that has decayed (remember the rule
#parent + #daughter = constant). Now you can determine the age as you did
before.
The long ages (billions
of years) given by radioactive dating of rocks seems an impossibly long time for
some people. Since radioactive rocks have been observed for only a few decades,
how do you know you can trust these long half-lifes and the long ages derived?
Here are some points to consider:
- The rate of decay should follow a simple exponential decline based on the
simple theory of probability in statistics. This same probability theory is
used to figure the odds of winning by gamblers.
- An exponential decay is seen for short-lived isotopes with half-lives of
only a few days.
- For the decades they have been observed, the long-lived isotopes also
follow an exponential decay.
- The decay probability should not depend on time because:
- An exponential decay IS observed for short-lived isotopes.
- Decays are nuclear reactions. Nuclear reactions only care about
size scales of 10-13 centimeters (100 million times smaller than
the wavelength of visible light). The composition and state of the
surrounding material will not affect the rate of decay.
- The
laws of nature or physics at the nuclear level should not change with
time.
- Astronomical observations show that the laws of physics are the same
everywhere in the universe and have been unchanged for the past 15 billion
years.
Some asteroids have
orbits that cross the orbit of the Earth. That means that the Earth
will be hit sometime. Recent studies have shown that the Earth has been
hit an alarmingly large number of times in the past. One large impact is now
thought to have contributed to the quick demise of the dinosaurs about 65
million years ago. What would be the effects of an asteroid hitting the Earth?
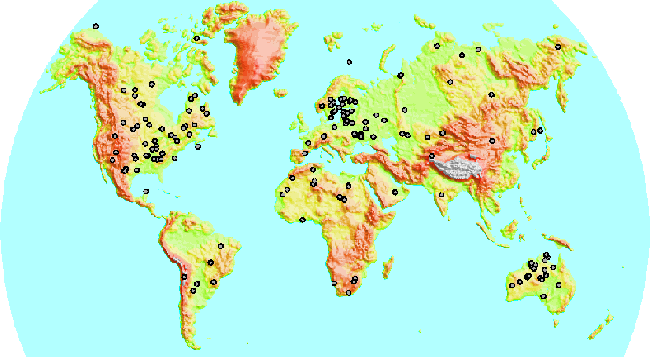
Known impact sites on the Earth's continents.
What follows is a condensation of an excellent article by Sydney van den
Bergh called ``Life and Death in the Inner Solar System'' in the May 1989 issue
of the Publications of the Astronomical Society of the Pacific (vol.
101, pages 500-509). He considers a typical impact scenario of a
10-kilometer object with density = 2.5 times that of water, impacting at a speed
of 20 kilometers/second. Its mass = 1.31 trillion tons (1.31 ×
1015 kilograms). A 1-kilometer object has a mass = 1.31
billion tons.
Obviously, something this big hitting the
Earth is going to hit with a lot of energy! Let's use the energy unit of 1
megaton of TNT (=4.2× 1015 Joules) to describe the energy of the
impact. This is the energy one million tons of dynamite would release
if it was exploded and is the energy unit used for nuclear explosions. The
largest yield of a thermonuclear warhead is around 50--100 megatons. The kinetic
energy of the falling object is converted to the explosion when it hits. The
10-kilometer object produces an explosion of 6 × 107 megatons of TNT
(equivalent to an earthquake of magnitude 12.4 on the Richter scale). The
1-kilometer object produces a milder explosion of ``only'' 6 × 104
megatons (equivalent to an earthquake of magnitude 9.4 on the Richter scale).
On its way to the impact, the asteroid pushes aside the air in front of it
creating a hole in the atmosphere. The atmosphere above the impact site is
removed for several tens of seconds. Before the surrounding air can rush back in
to fill the gap, material from the impact: vaporized asteroid, crustal material,
and ocean water (if it lands in the ocean), escapes through the hole and follows
a ballistic flight back down. Within two minutes after impact, about
105 cubic kilometers of ejecta (1013 tons) is lofted to
about 100 kilometers. If the asteroid hits the ocean, the surrounding water
returning over the the hot crater floor is vaporized, sending more water vapor
into the air.
There will be a crater regardless of where it lands. The diameter of
the crater in kilometers is = (energy of
impact)(1/3.4)/106.77. Plugging in the typical impact
values, you get a 150-kilometer diameter crater for the 10-kilometer asteroid
and a 20-kilometer diameter crater for the 1-kilometer asteroid.

Meteor (Barringer) Crater in northern Arizona (about 1
kilometer across). |
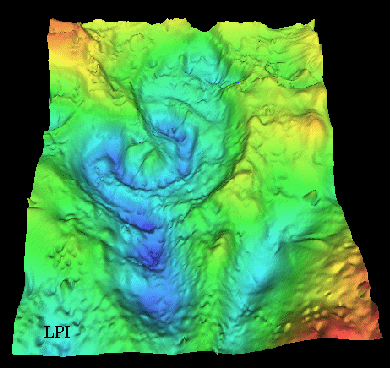
Chicxulub Crater in Yucatan, Mexico (from the one that may
have killed off the dinosaurs). |
The oceans cover about 75% of the Earth's
surface, so it is likely the asteroid will hit an ocean. The amount of water in
the ocean is nowhere near large enough to ``cushion'' the asteroid. The asteroid
will push the water aside and hit the ocean floor to create a large crater. The
water pushed aside will form a huge tidal wave, a tsunami. The tidal wave height
in meters = (distance from impact)-0.717 × (energy of
impact)0.495/ (1010.17). What this means is that a 10-km
asteroid hitting any deep point in the Pacific (the largest ocean) produces a
megatsunami along the entire Pacific Rim.
Some values for the height of the tsunami at different distances from the
impact site are given in the following table. The heights are given for the two
typical asteroids, a 10-kilometer and a 1-kilometer asteroid.
| Distance (in km) |
10 km |
1 km |
| 300 |
1.3 km |
43 m |
| 1000 |
540 m |
18 m |
| 3000 |
250 m |
3 m |
| 10000 |
100 m |
3 m |
The material ejected from the impact
through the hole in the atmosphere will re-enter all over the globe and heat up
from the friction with the atmosphere. The chunks of material will be hot enough
to produce a lot of infrared light. The heat from the glowing material will
start fires around the globe. Global fires will put about 7 ×
1010 tons of soot into the air. This would ``aggravate environmental
stresses associated with the ... impact.''
The heat from the shock wave of the
entering asteroid and reprocessing of the air close to the impact produces
nitric and nitrous acids over the next few months to one year. The chemical
reaction chain is:
- N2 + O2 > NO (molecular nitrogen
combined with molecular oxygen produces nitrogen monoxide)
- 2NO + O2 > 2NO2 (two nitrogen
monoxide molecules combined with one oxygen molecule produces two nitrogen
dioxide molecules)
- NO2 is converted to nitric and nitrous acids when it is
mixed with water.
These are really nasty acids. They will wash out of
the air when it rains---a worldwide deluge of acid rain with damaging effects:
- destruction or damage of foliage;
- great amounts of weathering of continental rocks;
- the upper ocean organisms are killed. These organisms are responsible for
locking up carbon dioxide in their shells (calcium carbonate) that would
eventually become limestone. However, the shells will dissolve in the acid
water. That along with the ``impact winter'' (described below) kills off about
90% of all marine nanoplankton species. A majority of the free oxygen from
photosynthesis on the Earth is made by nanoplankton.
- The ozone layer is destroyed by O3 reacting with
NO. The amount of ultraviolet light hitting the surface increases,
killing small organisms and plants (key parts of the food chain). The
NO2 causes respiratory damage in larger animals. Harmful
elements like Beryllium, Mercury, Thallium, etc. are let loose.
All of the dust in the air from
the impact and soot from the fires will block the Sun. For several months you
cannot see your hand in front of your face! The dramatic decrease of sunlight
reaching the surface produces a drastic short-term global reduction in
temperature, called impact winter. Plant photosynthesis stops and the
food chain collapses.
The cooling is followed by a much more prolonged period of increased
temperature due to a large increase in the greenhouse
effect. The greenhouse effect is increased because of the increase of
the carbon dioxide and water vapor in the air. The carbon dioxide level rises
because the plants are burned and most of the plankton are wiped out. Also,
water vapor in the air from the impact stays aloft for awhile. The temperatures
are too warm for comfort for awhile.
Astronomers have requested funding for an observing program called Spaceguard to catalog
all of the near-Earth asteroids and short period comets. Of the approximately
2000 asteroids that are thought to pose a threat of impacting Earth, less than 10% have been found
so far. The international
program would take 10 years to create a comprehensive catalog of all of the
hazardous asteroids and comets. The cost for the entire program (building six
special purpose telescopes and operation costs for ten years) would be less than
what it costs to make a popular movie like Deep Impact or
Armageddon. Select the hot links in this paragraph to find out more about
Spaceguard. Another
nice site is the Asteroid and
Comet Hazards site at NASA.
Vocabulary
| carbonaceous meteorite |
chondrules |
differentiated |
| half-life |
isotope |
meteorite |
| primitive |
radioactive dating |
- Where are most of the asteroids found?
- Why are spacecraft able to pass through the asteroid belt without getting
hit?
- What are the three types of asteroids and what are they made of?
- Why are more meteors seen after midnight?
- What are the proportions of the three types of meteorites and what are
they made of?
- What type of asteroid does each type of meteorite correspond to?
- Which meteorites are primitive and why are they particularly
important for understanding the origin of the planets?
- What makes carbonaceous meteorites so special?
- Why are chondrules especially important for solar system formation
models? What type of meteorite are they likely to be found in?
- How old are the various types of meteorites and why are they used to find
the age of the solar system?
- Why do iron meteorites make up 40% of the ``finds'', but are only 2 to 3%
of the total meteorites? What causes the biasing of the ``finds''?
- Why is Antarctica a good place to get an unbiased sample of meteorites?
- What makes SNC meteorites so unique and how are they different than other
types of meteorites?
- Why are iron and stoney-iron meteorites thought to come from a
differentiated body?
- Does radioactive dating give us relative or absolute ages for
rocks? What type of ``age'' does it tell us?
- How do you use the half-life to find the age of radioactive rocks?
- If the half-life of a radioactive sample is 1 month, is a sample of it
completely decayed after 2 months? If not, how much is left?
- Uranium-235 has a half-life of 700 million years. How long will you have
to wait until a 1-kilogram chunk decays so that only 0.0625 kilograms (1/16
kg) is left?
- How are the very old ages derived for some radioactive rocks known to be
correct?
- If a large asteroid were to hit Earth, how much of the Earth's surface
would be affected?
 Go
to Earth-Venus-Mars section
Go
to Earth-Venus-Mars section
 Comets and
solar system formation sections
Comets and
solar system formation sections
last update: 18 February 1999
Is this page a copy of
Strobel's Astronomy Notes?
Author of original content:
Nick Strobel











![]() Go
to Earth-Venus-Mars section
Go
to Earth-Venus-Mars section ![]() Comets and
solar system formation sections
Comets and
solar system formation sections